A Comparison of Red Fluorescent Proteins to Model DNA Vaccine Expression by Whole Animal In Vivo Imaging
PLOS One, June 2015
Vaccines are an incredibly potent tool in our arsenal to prevent infections. Simplistically they work by exposing the immune system to a safe part of the infectious bacteria or virus and evoking a memory response. When the vaccinated individual encounters the actual bug in real life, this memory allows the immune system to recognise and respond to the bug faster, thus preventing the infection before it takes hold. A critical step in vaccine development is the identification, isolation and mass production of the parts of the virus and bacteria that are able to evoke an immune memory. These components are often made up of proteins, which can be problematic and expensive to make to a standard that is acceptable for use as a medicine. Recently an alternative approach has been developed called DNA vaccination, which we describe in this review. DNA, the material of genes, contains the coding message for the production of proteins by cells. In the 1990’s a number of groups made the breakthrough observation, that if you inject DNA encoding proteins from a pathogen into a mouse, the mouse will make the protein in its cells and generate an immune response to this protein. This technique has the potential to significantly change vaccines because it is cheaper, easier and faster to make, for example, if a new viral epidemic occurs e.g. Ebola or MERS-CoV the time to make and test a DNA vaccine could be significantly shorter and therefore the spread of the disease would be reduced.
 |
| The rainbow explosion of fluorescent proteins, From Roger Tsien's Nobel Prize Talk. |
However, DNA vaccines have performed poorly in early phase
clinical trials (i.e. they work really well in mice, but poorly in people). There
have been a number of reasons proposed for this failure, but one possibility is
that, after immunisation, the genes encoded by the DNA vaccine are not turned
into protein by the patient (technically referred to as poor expression). In our recent paper, we
set out to address this problem in our study, using a novel technology called
in vivo imaging, which uses the proteins that allow jellyfish to glow in the
dark and fireflies to flash at night. The original fluorescent protein was
green (named green fluoresecent protein), but since then a whole colour chart
of proteins has been developed with names like tdTomato and mPlum. If the
proteins are injected into mice, they can, using a special camera, be seen. The
colour of the protein has a significant impact on our ability to see it in
mice, blue and green proteins work poorly because the skin has evolved to
reflect light in these wavelengths as a defence against damage caused by UV
light. Red proteins tend to be better and in our recently published paper we
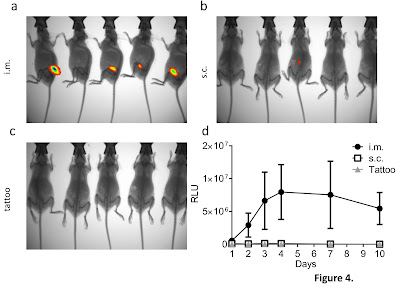
tested a range of red proteins delivered as DNA, called tdTomato, mCherry, mKatushka
and tdKatushka. We observed that only tdTomato gave us a strong enough signal
to see in the mice and that there are number of confounding problems with the
approach – pens we use to identify individual mice fluoresce in the same colour
as mouse poo.
 |
| An Unusual Location for Scientific Training! |
Delivering the DNA by different routes also had an impact on
the brightness with DNA injected into muscles producing a brighter signal than
DNA injected into the skin. We also looked into alternate methods of delivering
vaccines trying to more directly access cells involved in the immune response.
One approach we tried was tattooing, which is exactly as it sounds, we tattooed
DNA directly onto the skin of mice using the same system as you might get an
anchor or ex-girlfriend’s name tattooed on your arm. This came as a kit and to
get training, Katya (the lead author) had to get help from the local tattoo
parlour on Praed St! Delivering the DNA that expressed the red
proteins by tattoo failed to induce any signal, but when we used it to deliver
a vaccine, the mice were protected against influenza infection. However, this
result needs some caution as the equivalent body surface area in a person would
be the entire upper thigh!
 |
| Visualising Luciferase transfection in vivo |
This work was supported by the Medical Research Council
(MRC) as part of a CASE studentship (collaborative awards in science and
engineering). These awards are a collaboration between academia and industry,
with the industrial partner providing a novel product or technology and
expertise in that area and the academic providing the capacity to perform in
depth, slightly more esoteric research. The scheme is mutually beneficial as
the academic partner gets a student to pursue novel areas and the industrial
partner gets to explore angles that would not necessarily fit within the usual
timeline constraints of getting products to market. We have been collaborating
with a biotech startup called Touchlight Genetics who have a novel
process for generating DNA without bacteria. As part of this collaboration, we
have previously shown that the DNA is as effective as conventional bacteriaderived DNA and we are working to develop new vaccines with the
technology. Overall, we conclude that imaging protein expression from DNA may
be useful for some applications, but is poorly predictive of vaccine efficacy.
No comments:
Post a Comment