There’s no shame in not knowing the names of every bacteria,
there are billions if not trillions of different bacterial species. The number
that are pathogenic in humans is significantly lower – probably in the high
hundreds. One review estimates
that there are 1,400 known species of human pathogens (including viruses and
parasites). So, it is still ok to not have heard of all of them.
One that probably passes under the radar of many people is Acinetobacter
baumannii, not least because it is an absolute nightmare to spell. It is a
gram-negative opportunistic pathogen. Most cases of A. baumannii
infection are hospital acquired, often catheter or ventilator associated. There
are approximately 1 million A. baumannii infections annually and it is
associated with a nearly 35% mortality rate. The most pressing problem associated
with A. baumannii is antibiotic resistance; it is extremely drug
resistant – with nearly half of the infections resistant to the last line
antibiotic carbapenem. In fact Acinetobacter is the A in ESKAPE (the priority
list of the most important antibiotic resistant bacteria).
Since it is so drug resistant, other strategies are needed
to control it. One of which might be vaccines. In our recent study - Intranasal
immunization with outer membrane vesicles (OMV) protects against airway
colonization and systemic infection with Acinetobacter baumannii we developed
a novel vaccine against this important pathogen. The approach we used was to
manipulate a product of the bacteria itself to generate protection. A.
baumannii produces little bubbles of lipids and proteins called outer
membrane vesicles (OMVs). Bacteria use these OMV to communicate with each other
and potentially help them better infect us. However, they also contain lots of
different bacterial antigens, some of which are potentially protective. The highly
effective vaccine against Meningitis B (Bexsero made by GSK) contains OMV.
In our study, we isolated OMV from clinical isolates of A.
baumannii. Using clinically derived strains was important – as these are
probably closer to the ones in circulation, than the ones used in many studies
from older varieties. To test whether the OMV worked, we developed new models
of infection using the same strains. We showed the recent clinical isolates
were more pathogenic than the standard lab strain. The clinical isolates were
able to escape from the lungs into the blood and appeared to stably colonise
the upper airways for at least 7 days after the initial infection.
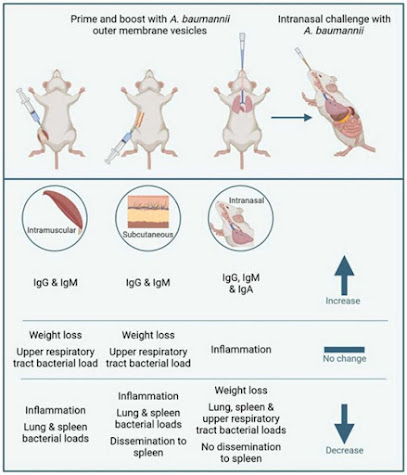
Our first studies tested injecting the vaccine into the muscle
– as this is the most common route of vaccination used. OMV injected this way
did give a good immune response – leading to the induction of antibodies.
However, the immunity raised following this route of immunisation was not very
protective against subsequent infection. When area of interest in the vaccine
field has been mucosal vaccination – delivering vaccines to the site of infection,
in this case the nose and lungs. When Dr Sophie Higham (lead author on the
paper) immunised via the nose, protection was significantly improved with a
dramatic reduction in bacterial load following infection.
This work shows two things – OMV can be very effective vaccine candidates against bacterial infections and that immunising in the site of infection can be beneficial. The next step is to look how to scale up for human studies.